Abstract
Background: Differentiation arrest resulting from aberrant methylation represents a key pathogenic and phenotypic feature of MDS. Hypomethylating agents (HMA) can reverse epigenetic dysregulation, but as single agents fail to control the disease in approximately 50% of patients, many of whom progress to AML and carry a dismal prognosis. We hypothesized that Differentiation Scoring (DS) derived from Cellworks Computational Omics Biology Model (CBM) of each patient's genomic aberrations could serve as a biomarker to predict response to HMA.
Methods: We studied 169 MDS patients treated with azacytidine (n = 86) or decitabine (n = 83) whose genomic aberrations and clinical outcomes were available from public databases. Biosimulation of the disease state was computationally derived from pathways that were up- or down-regulated by individual genomic aberrations. DS was defined as the ratio of the quantified impact of key differentiation biomarkers in myeloid malignancies (CEBPA, GATA1, SPI1 and ITGAM) divided by the impact of HOXA9 transcription, a known repressor of differentiation, and normalized on a log2 scale.
We also used CBM to determine the change in DS attributable to HMA to define an efficacy score (ES) as a parameter of drug response. A Pearson correlation coefficient was obtained using the two variables. DS in clinical responders (R) and non-responders (NR) was evaluated with the Student t-test. We defined DS > 1.5 as DS-high and < 1.5 as DS-low. Survival data were available for 101 patients and correlated with DS-high (n=49) and DS-low (n=52) groups using a KM-curve.
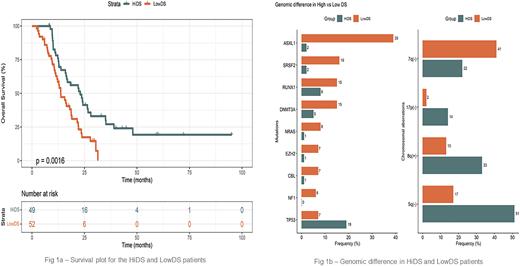
Results: Median age of this cohort was 69 years (range, 20-87 years), including 56 females, 111 males, and 2 of unknown sex. Regarding HMA response, 62 patients were R while 107 were NR. Prior to therapy, 86 patients had DS-high and 83 had DS-low scores. Post-treatment DS were correlated with HMA ES (R = 0.58, p < 2.2e-16), and showed a favorable HMA response for DS-high patients. Biosimulation showed the incremental benefit on DS of HMA was greater for people with DS-high than DS-low scores (44% vs 16%, p = 7.51 e-17). Median DS was higher in R than NR (p=0.00232). Patients with DS-high scores had superior survival compared to those with DS-low scores (log rank p = 0.0016). (Figure 1a)
Different genomic aberrations contributed to the DS scores. DS-high patients had more TP53 mutations, 17p del, 8q amp, and 5q del. On the other hand, DS-low patients more often had mutations in ASXL1, SRSF2, RUNX1, DNMT3A, EZH2, NF1, NRAS, CBL, or 7q deletion. (Figure 1b)
Loss of TP53 due to mutation or deletion upregulates DNMT1 and silences HOXA9 transcription, leading to DS-high scores. KAT6A (8q) amp also upregulates EZH2 and CpG methylation, while 5q del causes GATA1 transcription, increasing CpG methylation via SALL4 to produce high ß-catenin signaling that led to DS-high scores. By contrast, 7q loss, ASXL1, SRSF2, and EZH2 mutations downregulated EZH2, diminished CpG methylation and unsilenced HOXA9 transcription. As a result, HOXA9 arrested differentiation results in DS-low scores. Additionally, NF1 or NRAS mutations upregulate ERK signaling which inhibits CEBPA, and also caused DS-low scores.
Conclusions: Computational biosimulation based on patients’ individual genomic aberrations reveals a spectrum of DS among patients with MDS. DS strongly predicted HMA response. In general, NR had lower DS and survival than DS-high patients. While patients with DS-low scores might have fared even worse without HMA therapy, we propose that DS-low patients should be considered for novel combination therapy at the time of diagnosis given their unsatisfactory outcomes with HMA therapy as a single agent.
Disclosures
Castro:Cellworks Group Inc.: Current Employment, Current equity holder in private company; Neurovigil: Current equity holder in private company, Other: Scientific Advisory Board; Bugworks: Consultancy; Exact Sciences Inc.: Consultancy. Usmani:Cellworks Group Inc.: Current Employment. Kumar:Cellworks Group Inc.: Current Employment. Chauhan:Cellworks Group Inc.: Current Employment. Kapoor:Cellworks Group Inc.: Current Employment. Khandelwal:Cellworks Group Inc.: Current Employment. Patil:Cellworks Group Inc.: Current Employment. Mohapatra:Cellworks Group Inc.: Current Employment. Tyagi:Cellworks Group Inc.: Current Employment. Sauban:Cellworks Group Inc.: Current Employment. Nair:Cellworks Group Inc.: Current Employment. Shyamasundar:Cellworks Group Inc.: Current Employment. Dutta:Cellworks Group Inc.: Current Employment. Mitra:Cellworks Group Inc.: Current Employment. Gupta:Cellworks Group Inc.: Current Employment. Prasad:Cellworks Group Inc.: Current Employment. Agrawal:Cellworks Group Inc.: Current Employment. Balla:Cellworks Group Inc.: Current Employment. Suseela:Cellworks Group Inc.: Current Employment. Balakrishnan:Cellworks Group Inc.: Current Employment. Promod:Cellworks Group Inc.: Current Employment. Patel:Cellworks Group Inc.: Current Employment. Macpherson:Cellworks Group Inc.: Current Employment. Wingrove:Cellworks: Current Employment, Current equity holder in private company. Howard:Cellworks Group Inc.: Consultancy; Servier: Consultancy; Sanofi: Consultancy, Other: Speaker fees. Marcucci:Abbvie: Other: Speaker and advisory scientific board meetings; Agios: Other: Speaker and advisory scientific board meetings; Novartis: Other: Speaker and advisory scientific board meetings.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal